European study to protect preterm newborns from hospital-acquired infections launches at NNUH
The NeoDeco study, sponsored by Fondazione Penta ETS, is investigating whether skin-to-skin contact can help reduce infection rates in neonatal intensive care units (NICUs). Three hospitals in the United Kingdom, including the Norfolk and Norwich University (NNUH), are joining this Europe-wide trial to improve care for premature babies.
Hospital-acquired infections pose a serious risk for newborns across Europe, especially premature babies. To address this challenge, the NeoDeco study – sponsored by Fondazione Penta ETS and part of the EU-funded NeoIPC project, coordinated by the University of Padua – is investigating the effectiveness of Kangaroo Care as an innovative method to prevent infections in NICUs.
The study will involve up to 24 NICUs in Italy, Greece, Spain, Switzerland and the United Kingdom, including NNUH, bringing together hospitals from across Europe in a joint effort to improve neonatal health.
Professor Paul Clarke, NNUH Consultant Neonatologist and local Principal Investigator for the study, said: “For many years we have encouraged parents to carry out Kangaroo Care in our NICU because we know the benefits it brings, such as better bonding, attachment and growth, and increasing the confidence of parents in caring for their babies. There is now a large body of evidence that shows that babies, especially premature babies, are more stable and have better outcomes with this early direct skin-to-skin contact with their parents. We’re delighted to be taking part in this study to see if optimised kangaroo care can also reduce the risk of infections in babies. We are looking to recruit around 120 babies onto the study at NNUH. If the study shows that optimising the duration and quality of kangaroo care reduces the risk of infection, this will potentially help many thousands of other babies treated on NICUs around the world.”
Every year, around 400,000 babies in Europe are born preterm and require admission to a NICU. While neonatal intensive care greatly improves their chances of survival, it also exposes these vulnerable babies to antibiotic-resistant bacteria from the hospital environment. These bacteria can lead to severe infections, sepsis, and even outbreaks of disease, posing significant risks in the fragile NICU environment.
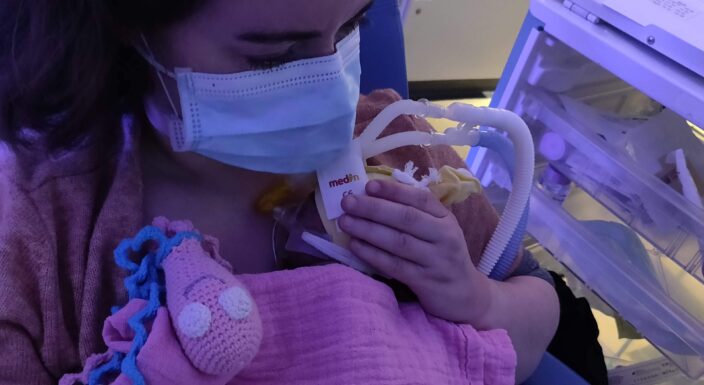
For premature newborns, the World Health Organization recommends Kangaroo Care: a simple, safe and low-cost practice that involves prolonged skin-to-skin contact between the baby and their caregiver. Kangaroo Care is thought to help babies acquire more healthy bacteria, which can boost the development of their immune systems.
Despite its benefits, the broader impact of Kangaroo Care on preventing transmission of antibiotic-resistant bacteria and reducing NICU infections is still underexplored. “Comprehensive studies on the effects of Kangaroo Care on neonatal infections caused by difficult to treat bacteria at NICU-wide level are currently lacking”, added Julia Bielicki, Professor in Paediatric Infectious Diseases at City St George’s, University of London and NeoDeco Chief Investigator.
“NeoDeco’s objectives extend beyond examining Kangaroo Care’s impact on individual babies. Our focus is to evaluate its potential as an infection prevention and control measure, when applied to most babies in an optimal and sustainable manner by NICUs”, Bielicki adds.
“Optimised” Kangaroo Care is defined as early, repeated and sustained skin-to-skin contact, following international best practice recommendations. While some European NICUs already practise Kangaroo Care, the extent of its implementation varies widely in terms of how early, how often, and how long it is practised.
To support NICUs in achieving the optimal levels of Kangaroo Care required by the study, a team of implementation science experts from the University of Zurich will provide tailored site-level support to ensure its successful adoption. By working closely with NICU staff, the team will assist hospitals in overcoming potential challenges, such as the preferences and beliefs of parents and healthcare professionals, ensuring the effective implementation of optimised Kangaroo Care.
Carlo Giaquinto, Professor of Paediatrics at the University of Padua (Italy), and President of Fondazione Penta ETS said: “If successful, NeoDeco will not only contribute to validate the health benefits of Kangaroo Care but could also establish a new standard of care to improve preterm birth outcomes across Europe, ensuring a healthier start for this vulnerable population.”
NeoDeco launched in June 2024 with an initial group of ten NICUs from Greece and Switzerland. Eleven neonatal units from Greece, Italy, Spain and the United Kingdom have joined the study between February and March 2025, with additional units expected to join soon. This could bring the total to 24 sites across five European countries. Results from the study will be available at the end of 2026.
About NeoIPC
NeoIPC is a project led by the University of Padua (Italy) that seeks to foster infection prevention and control research and implementation in the high-risk setting of neonatal intensive care. NeoIPC will achieve this through engaging units in an innovative approach towards the evaluation and implementation of IPC measures to reduce resistant bacterial infection in this setting. For more information, visit https://neoipc.org/.
Project partners are: University of Padua, Italy; Fondazione Penta ETS, Italy; City St George’s, University of London, United Kingdom; Universitair Medisch Centrum Utrecht, The Netherlands; European Clinical Research Alliance for Infectious Diseases, The Netherlands; Universität Zürich, Switzerland; Universitäts-Kinderspital beider Basel, Switzerland; Tartu Ulikool, Estonia; Schweizerisches Tropen und Public Health Institut, Switzerland; Aristotelio Panepistimio Thessalonikis, Greece; Universiteit Antwerpen, Belgium; Charité – Universitätsmedizin Berlin, Germany; Osakidetza – Servicio Vasco de Salud, Spain; Stellenbosch University, South Africa.
NeoIPC has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 965328. The contents of this press release are the sole responsibility of NeoIPC and do not necessarily reflect the opinion of the European Union.
About NeoDeco
NeoDeco is a pragmatic, multicentre, parallel group, cluster randomised hybrid effectiveness-implementation study with baseline assessment, wash-in period and staggered randomisation. It is sponsored by Fondazione Penta ETS, Italy. NICUs that are currently part of the study are:
- Greece: Aglaia Kyriakou Children’s Hospital, Athens; University General Hospital Attikon, Athens; University Hospital of Heraklion; Ioannina University Hospital, Ioannina; General University Hospital of Patras; Hippokration Hospital, Thessaloniki; Papageorgiou Hospital, Thessaloniki
- Italy: Azienda Ospedaliero-Universitaria S. Anna di Ferrara; Ospedale Universitario Policlinico Paolo Giaccone, Palermo; Ospedale San Bortolo di Vicenza
- Spain: Hospital General Universitario, Alicante; Hospital Universitario Cruces, Bilbao; Hospital Materno-Infantil del Hospital Universitario Regional de Málaga
- Switzerland: University Children’s Hospital Basel; Geneva University Hospitals; Children’s Hospital of Eastern Switzerland, St. Gallen; University Hospital of Bern; Universitätsspital Zürich – University Hospital Zurich
- United Kingdom: University Hospitals Coventry and Warwickshire, Coventry; George’s University Hospitals NHS Foundation Trust, London; Norfolk and Norwich University Hospitals NHS Foundation Trust, Norwich.
For more information, visit https://neoipc.org/neodeco/.
About Penta
Penta is an international independent scientific network devoted to advancing research on optimising the prevention, diagnosis, and treatment of infectious diseases in children, globally. More than 30 years since its creation, Penta is today one of the most prominent scientific organisations dedicated to research on maternal and childhood infections, such as HIV and viral infections, fungal infections, respiratory infections, and severe bacterial infections. www.penta-id.org


